- Messages
- 712
- Reactions
- 177
Sounds like you pretty much know what you're talking about. In any case - this is a horrible mess with long lasting effects for at least the local people, and maybe the rest of us as well.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
Crazy as it sounds - a google search indicates that these reactors have the containment pools ABOVE the reactor... (multiple sources) But conflicting reports as to whether they have been blown.
Sounds like you pretty much know what you're talking about. In any case - this is a horrible mess with long lasting effects for at least the local people, and maybe the rest of us as well.
i'll see if i can dig up a diagram in a bit.
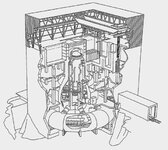
they're NOT above the reactor- they're to the side, and guarded by steel and concrete framing. the actual tops of the buildings- the "attics," essentially, are the secondary containment "vessels" for each reactor. they're designed to take flash steam, hydrogen in the event of an emergency, and blow up if it comes down to it. while the hydrogen explosions have been gnarly and damaging, the area where the pools are kept are well below the blast zone.
i'll see if i can dig up a diagram in a bit.
Why A Nuclear Reactor Will Never Become A Bomb

By Alasdair Wilkins on March 17, 2011 at 10:14 AM
As Japan's Fukushima power plant continues to struggle with massive equipment failure and radiation release that could well reach Chernobyl levels, we can take some small comfort in the knowledge that a full-on nuclear explosion is completely impossible. Here's why.
Chain Reactions
Both nuclear reactors and nuclear weapons depend upon chain reactions. Such reactions require the presence of fissile materials, which are any atomic isotopes which can, when they undergo a particular nuclear reaction, create the raw materials necessary for the same reaction to repeat itself. There's only one naturally occurring fissile isotope, and that's uranium-235 – all other fissile isotopes, such as various plutonium isotopes, have to be artificially "bred" from natural isotopes.
So how does a chain reaction work? Let's consider the one involving uranium-235, which is the chain reaction used in nuclear reactors and many nuclear weapons. A free neutron hits a slow-moving uranium-235 isotope and is absorbed into it. Here one of two things can happen: the uranium will fission into two lighter, faster-moving isotopes, typically krypton-92 and barium-141, as well as some gamma radiation. The nuclear reactor is then able to absorb this energy, which is about three million times the energy the same amount of coal can produce in conventional burning.
Crucially, this reaction also creates additional free neutrons, which can then be absorbed into other uranium-235 isotopes and start the whole process over again. This is why, of the naturally occurring uranium isotopes, only uranium-235 is fissile – when uranium-238 undergoes such reactions, it can't release neutrons with the energy to start up a chain reaction.
As long as the reactions create an average of one or more neutrons, the chain reaction can go on indefinitely. Frequently, these reactions create more than one free neutron, which can cause the amount of energy being produced to increase over subsequent generations.
Safety Measures
To prevent a potentially dangerous buildup of energy, nuclear reactors build in huge numbers of fail-safes and redundancies. One of the better-known methods is the use of control rods, which are made from materials such as boron that absorb neutrons but cannot undergo nuclear reactions. In the event of a runaway energy buildup, these rods are often rigged to fall right into the heart of the reactor to absorb all the free neutrons and shut down the chain reaction. Mismanagement of these rods was one of many factors behind the Chernobyl disaster.
And yes, if all the fail-safes and redundancies do somehow fail to stop the heat buildup – as they did in Chernobyl, as they partially did at Three Mile Island, and as they might do in the current situation in Japan – there can be some pretty nasty effects. The most infamous threat is that of a nuclear meltdown, which is when the heat buildup causes the entire core to melt, damaging the protective structures to the extent that intensely radioactive materials can be released into the environment.
A meltdown obviously can have horrific short-term and long-term environmental effects, but what about an actual explosion? Could a nuclear reactor explode with the sort of force unleashed in the bombings of Hiroshima and Nagasaki? After all, Chernobyl exploded, didn't it? Thankfully, the answer to all this is no, a nuclear explosion is impossible, and the destructive blast at Chernobyl was actually just a steam explosion – and a good thing too, because a nuclear blast of the same magnitude could have turned Chernobyl from a horrific disaster to a full-on cataclysm. But again, such an explosion is totally impossible, and to understand why we have to look at the difference between nuclear reactors and nuclear weapons.
A Matter of Quality
Although we think of uranium as the most common fuel for nuclear reactions, that isn't strictly true. Natural uranium is pretty much completely useless for nuclear reactors, let alone nuclear weapons. This is because natural uranium is about 99.3 per cent composed of the isotope uranium-238, while just .7 per cent uranium-235, and only the latter is capable of sustaining nuclear chain reactions.
To make uranium usable for chain reactions, it needs to be enriched. This involves carefully separating out the uranium-235 from the uranium-238. The two have practically the same mass, particularly because uranium-235 is typically found in a compound state with fluorine, which nudges its mass to pretty much that of its big brother.
Nuclear reactors need low-enriched uranium, which is defined as anything with less than a 20 per cent concentration of uranium-235. Typically, nuclear power plants only need a 3-4 per cent concentration to have reactor grade uranium. Nuclear weapons, on the other hand, require highly enriched uranium for the sort of runaway chain reaction that can create a nuclear explosion. The cutoff for high enrichment is just 20 per cent, but the vast majority of nuclear weapons use uranium with a concentration of anywhere from 80 to 95 per cent. The bomb dropped on Hiroshima, for instance, used 80 per cent enriched uranium.
Critical Mass
So what's the real difference between low- and high-enriched uranium? Why couldn't low-enriched uranium create an explosion that's just not quite as severe as its high-enriched equivalent? For that, we must turn to another term that is frequently mentioned but infrequently understood, and that's critical mass. The term simply means that there's enough fissile material present to sustain a chain reaction, and a supercritical mass is where enough material is present for the fission rate to increase.
Although mass is obviously an important factor here – hence the name – it's possible to alter the point of criticality by varying other attributes of the material, including shape and density. A nuclear weapon is designed to release all its energy in one incredibly destructive blast, which means the material wants to be as densely packed with fissile material as possible, and the material should be packed into as homogeneous a sphere as possible.
That's absolutely nothing like the design of reactor cores, which is meant to produce a steady, controlled release of energy, and even the sort of energy buildup needed to produce a meltdown can't ever attain the speed and intensity needed for an explosive nuclear energy release. The geometric arrangement of uranium-235 in a nuclear reactor is just fundamentally not conducive to the spherical arrangement needed for an explosive chain reaction, and the amount of non-fissile uranium-238 in reactor-grade uranium also stops any runaway reactions dead in their tracks.
Why this matters
None of this is intended to minimise the very real dangers of nuclear reactor accidents. As seen in Chernobyl, meltdowns can have absolutely devastating environmental effects, and the nearby town of Pripyat remains uninhabitable twenty-five years after the accident. We don't yet know whether the current situation in Japan will reach Chernobyl levels – experts have at least seriously considered the possibility, but we still don't have a clear handle on the full extent of the danger.
Still, even in the midst of disasters of unimaginable proportions, it's crucial to try to maintain some nuance regarding the line between real fears and hysteria, and in the case of nuclear safety the best way to do that is to understand a little of the science behind the technology. The threat of a nuclear meltdown is worrisome enough without having to invoke the specter of the mushroom cloud.
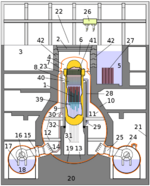

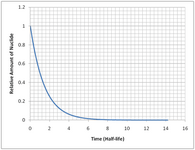
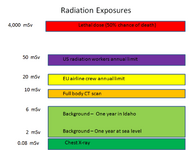

and lo and behold.... no nuclear holocaust. whatever will i put my energy into now, now that the nuclear zombie uprising will not be happening?
guess i'll go get the .50 off the roof, pack up the claymores, and sweep up all those caltrops...